
New Data For Moderate Patients
New data for moderate patients – SAVANT clinical trial sub-analysis from Dr. Meuth
Tetrahydrocannabinol and cannabidiol oromucosal spray in resistant multiple sclerosis spasticity: consistency of response across subgroups from the SAVANT randomized clinical trial
ORIGINAL CLINICAL TRIAL OBJECTIVES:
To determine whether the severity of resistant MS spasticity patients ́ disability status, spasticity or spasticity duration at the start of treatment might influence MS spasticity improvement possibilities.
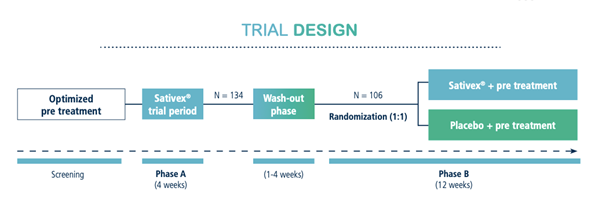
STUDY DESIGN:
Prospective, parallel-group, randomized, double-blind, placebo-controlled two-phase trial with 210 MS patients
NEW SUBGROUP ANALYSIS KEY ENDPOINTS:
- Evolution in mean MS spasticity 0-10 NRS scores and mean pain 0-10 NRS scores in the ITT population was analyzed from double-blind treatment data in subgroups of patients stratified according to:
- EDSS score < 6 (ambulatory) or ≥ 6 at randomization.
- Spasticity 0-10 NRS score ≤ 6 (4-6: moderate spasticity) or > 6 (severe spasticity) at randomization or
- Years with MS spasticity (≤ 5 or > 5 years) at randomization.
- Least squares (LS) mean change from baseline to week 12 randomized phase of the clinical trial (phase B).
NRS, Numerical Rating Scale (spasticity 0-10 NRS score).
EDSS, Expanded Disability Status Scale.
MS, multiple sclerosis.
RESULTS:
- THC:CBD oromucosal spray halved mean severity scores for spasticity and pain in all subgroups.
- Statistical comparisons in these post-hoc analyses were affected by the limited sample size at certain subgroups
- Active treatment significantly improved mean spasticity severity scores versus placebo from week 4 onwards in both EDSS subgroups, in the severe spasticity subgroup, and in both spasticity duration subgroups.
- Active treatment significantly improved mean pain severity scores versus placebo in the >6 EDSS subgroup, in the severe spasticity subgroup and in both spasticity duration subgroups.
CONCLUSION:
- Add-on THC:CBD oromucosal spray consistently relieves resistant spas- ticity across subgroups defined by baseline EDSS score, spasticity severity NRS score and spasticity duration.
- Patients with moderate resistant MS spasticity benefit numerically from treatment; patients with severe resistant spasticity achieve significant therapeutic gains.
- Spasticity-associated pain often improves similarly in the same subgroups.
- Markova J, Essner U, Akmaz B, et al. Sativex as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci. 2019 Feb;129(2):119-128.
- Meuth S, Henze T, Essner U, Trompke C, Vila C. Tetrahydrocannabinol and cannabidiol oromucosal spray in resistant multiple sclerosis spasticity: predictors of response from the SAVANT randomized clinical trial. [Unpublished; pending approval]. 2019.
NO-SAT-2000009
